What Is The Liquid In The Covid-19 Test Kit? The Answer Is…
To start with, what is the liquid in the covid-19 test kit?
Actually, sodium azide is present in some of the kits. Chemicals like sodium azide have the potential to be poisonous. They could be mistaken for eye drops. Children might accidentally touch their skin with it. Adults occasionally make the mistake of putting them in their eyes.
Please continue reading so I can go into more detail about what is liquid in the COVID-19 test kit.
Table of Contents
Covid-19 Test Kit Liquid Contains Poisonous Chemical
Las Vegas (KSNV) — Cases of COVID-19 are once again steadily increasing. We are reminded that the liquid in those kits can be poisonous as more of us rely on at-home test kits.
According to the Nevada Poison Center, the dropper contains sodium azide, a substance that the CDC classifies as potentially lethal.
It could damage the heart and the brain if consumed in the appropriate amount.
Six calls about exposure have already been made to the Nevada Poison Control Center, the majority of them following the delivery of the test kits to homes across the nation.
“According to Shireen Banerji, director of Nevada Poison Control, “one was not disposed of properly,” which allowed a child to come across the used, small dropper and suck on it in order to obtain some of the residue.”
The Nevada Poison Center advises users to discard all material after a test and to keep them out of the reach of children and animals, even though the amount of sodium azide in the test kits is probably not enough to put one in the hospital.
Features Of Covid-19 Test Kit Liquid
- For self-testing in vitro diagnostic use only
- Simple to Use with Easy to read results
- Approved for sale in the UK as a Home Test Kit
Covid-19 Tests Limitations
Even tests that adhere to safety and performance requirements set by the law cannot be completely trusted. Additionally, the findings are only applicable to that sample and that particular time period.
Inquire with the test’s provider first if you have questions about what a test result means or what to do or not do after learning the results; if your questions are still unanswered, consult your doctor or another healthcare provider.
It’s critical to comprehend the limitations of COVID-19 tests because an incorrect or poorly interpreted result can result in a false sense of security. For instance, if a test you use yields a false negative result—saying you do not have the virus despite having it—and you are infected with the virus, you could unintentionally infect others or forego receiving the necessary treatment.
A major reason for the limitations of antibody testing is that we still know very little about COVID-19 immunity. For illustration, we are unsure of the following:
- antibodies protect you from getting COVID-19 in the future
- antibodies stop you from passing the virus on to others
- having a negative antibody test means you have never been infected
The label or instructions included with the testing kit will contain information about the test’s reliability (also referred to as “sensitivity” in some cases).
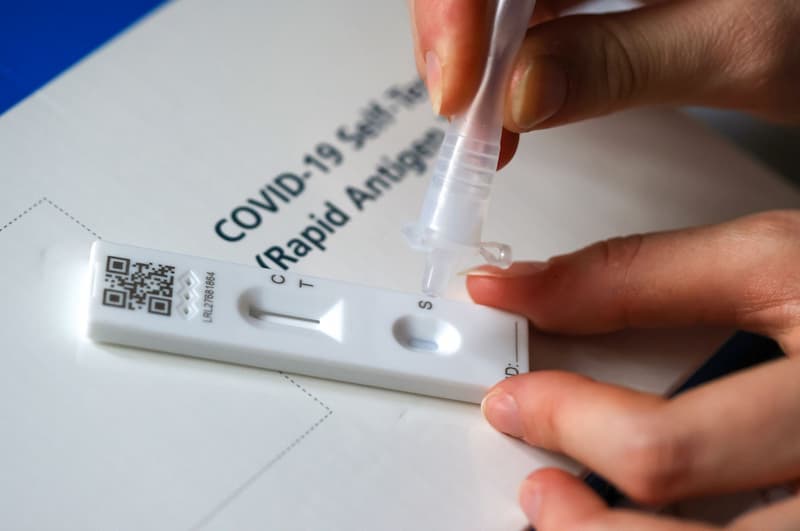
Covid-19 Testing Is Regulated
Both the COVID-19 tests and sample collection kits are medical devices. A valid CE, CE UKNI, or UKCA mark is necessary for a medical device to be safe to use. These certifications are the manufacturer’s acknowledgement that the test complies with the necessary performance and safety standards.
These regulatory requirements seek to ensure that the products are designed and produced to achieve the performance levels required by the manufacturer for the declared purpose and do not compromise the safety of users or patients.
The MHRA does not grant licenses for the CE, CE UKNI, or UKCA marks. The manufacturer affixes them to the item. Before a product can be sold in the UK, the manufacturer or their UK Responsible Person (UKRP) must register it with the MHRA.
Tests specifically used for self-testing must have been approved for CE/CE UKNI/UKCA marking by a Notified or Approved Body, or they must have received an Exceptional Use Authorization from the MHRA. On the packaging, a 4-digit identification number is located next to the CE mark symbol and a CE, CE UKNI, or UKCA mark designates this. tests for which an Exceptional Use Authorization has been granted.
A number of lateral flow antigen self-tests have now received CE certification from an EU notified body.
In order to guarantee the performance and safety of medical devices in the UK, the MHRA administers and enforces the country’s medical device laws. The agency also has a number of investigative and enforcement powers.
Final Words
What the liquid in the Covid-19 test kit is the subject of the post.
So, having read the post, do you now understand what the liquid in the COVID-19 test kit is? If you have any questions about the liquid in the covid-19 test kit, kindly leave a comment. I will answer right away.
I want to thank you for reading, but that’s not the end of it.

